Abstract
Introduction
Accurate and timely diagnosis of heparin-induced thrombocytopenia (HIT) is critical to patient management. Easy-to-perform platelet factor 4 (PF4)/heparin immunoassays have poor specificity while the reference standard test, serotonin release assay (SRA) is technically challenging, offered by few labs and dependent on the source of platelets used in testing. Recent data suggest that PF4-treated platelets express PF4 neoepitopes preferentially recognized by platelet-activating (pathogenic) HIT antibodies without any requirement for added heparin (Blood . 2015 Jan 1;125(1):155-61). The recently described PF4-dependent P-selectin expression assay (PEA), in which PF4-treated platelets are activated by pathogenic HIT antibodies, is based on this principle. Recent reports suggest that the PEA is more accurate (and sensitive) than the SRA for HIT diagnosis and for monitoring response to treatment (Chest . 2016 Sep;150 (3):506-15 & Padmanabhan et al. Chest. 2017 Apr 17 epub) and that it may detect pathogenic antibodies earlier than the SRA (Jones et al, Chest 2017 June 1 accepted). Here, we describe studies to define the operating characteristics of the PEA.
Methods
The PEA was performed as previously described using blood group O platelets pretreated with 37.5mcg/ml of PF4 (Chest . 2016 Sep;150(3):506-15). Serum samples from healthy controls and from patients with HIT, idiopathic thrombocytopenic purpura (ITP), thrombotic thrombocytopenic purpura (TTP) and post-transfusion purpura (PTP) samples were used as indicated in figure legends. A cut-off of 20% and 24% (with >50% inhibition with 100U/ml heparin) was considered positive in the SRA and PEA, respectively.
Results
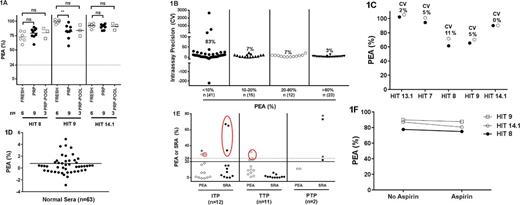
We examined PEA responses of fresh platelets from 6 donors and platelets isolated from platelet-rich plasma (PRP, 2-3 days old) of 9 blood donors using three HIT sera - a total of 45 unique serum-platelet combinations. Three samples containing pooled platelets (3 PRPs per pool) from the 9 PRP units were also studied. Fig 1A shows that there was little donor to donor variation in PEA responses of fresh platelets and slightly greater variation in the response of platelets from PRP of individual blood donors that was mitigated, however, when these platelets were pooled. Intra-assay precision was very high in the meaningful range of assay readout (>10% of maximum PEA expression) with coefficients of variation (CV) ranging from 3% to 7% when testing was done with 91 HIT-suspect samples (Fig 1B). Inter-assay CV was also small (Fig 1C). The PEA was highly specific and none of 63 healthy donor samples studied produced positive PEA test results (Fig 1D). Twenty-five patients suspected of thrombocytopenia due to non-HIT disease processes (ITP, TTP and PTP) were tested in the PEA and SRA. The two assays had identical false positive rates (3/25; Fig 1E). Of note, the three false positives detected by the PEA were at or just above the cut-off, while two of the 3 SRA false-positives were much stronger at >65% serotonin release. Finally, ingestion of aspirin had no apparent effect on platelet responsiveness in the PEA (Fig 1F).
Conclusions
The findings indicate that the PEA is robust, reproducible, specific and relatively unaffected by the choice of platelet donor. Studies also suggest that valid test results can be obtained with PRP-derived platelets stored for several days at room temperature. It is technically easy to perform with a run time of 2-3 hours, and has the potential to facilitate rapid and accurate HIT diagnosis, thus resulting in more timely and appropriate management.
Figure legend:
(1A). The PEA was performed with blood group O platelets from 6 freshly drawn donors (open circles), platelets obtained from 9 PRP bags (closed circles) and three pools of 3 PRPs each (open squares). HIT samples used are indicated on the abscissa. (1B) Intra-assay precision: Each of 91 HIT samples were run twice in the same experiment. (1C) Inter-assay precision: Each of 5 HIT sera were run against the same target platelets in two separate experiments. (1D) PEA results obtained with 63 healthy subjects is shown. (1E) PEA and SRA were performed on patients suspected of ITP, TTP and PTP. Asterisks indicate results greater than the positive cut-off in the PEA/SRA that were not inhibited by high dose heparin. Red circles indicate false-positive results. (1F) PEA performed with platelets from a donor before and 12 hours after 325mg aspirin ingestion is shown.
Jones: BloodCenter of Wisconsin: Patents & Royalties: A patent application has been filed related to the PF4-dependent P-selectin expression assay (Method of Detecting Platelet- Activating Antibodies That Cause Heparin-Induced Thrombocytopenia/Thrombosis; PCT/US14/62591).. Bougie: BloodCenter of Wisconsin: Patents & Royalties: A patent application has been filed related to the PF4-dependent P-selectin expression assay (Method of Detecting Platelet- Activating Antibodies That Cause Heparin-Induced Thrombocytopenia/Thrombosis; PCT/US14/62591).. Curtis: Ionis Pharmaceuticals: Consultancy. Aster: BloodCenter of Wisconsin: Patents & Royalties: A patent application has been filed related to the PF4-dependent P-selectin expression assay (Method of Detecting Platelet- Activating Antibodies That Cause Heparin-Induced Thrombocytopenia/Thrombosis; PCT/US14/62591)., Research Funding. Padmanabhan: Retham Technologies LLC: Equity Ownership; BloodCenter of Wisconsin: Patents & Royalties: A patent application has been filed related to the PF4-dependent P-selectin expression assay (Method of Detecting Platelet- Activating Antibodies That Cause Heparin-Induced Thrombocytopenia/Thrombosis; PCT/US14/62591).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal